Abstract
Background: Indolent non-Hodgkin lymphoma (iNHL), including follicular lymphoma (FL) and marginal zone lymphoma (MZL), is typically responsive to initial chemoimmunotherapy, but relapse is expected. Single-agent rituximab is FDA approved and frequently administered for patients with relapsed/refractory (R/R) low-grade or follicular CD20-positive B-cell NHL. Lenalidomide is an immunomodulatory agent with preclinical and clinical antilymphoma activity alone and in combination with rituximab. We compared the efficacy and safety of lenalidomide plus rituximab (R2) to rituximab (plus placebo) in patients with R/R iNHL.
Methods: AUGMENT (NCT01938001) is a multicenter, double-blind, randomized phase III study of R2 vs rituximab/placebo (control) in patients with FL grade 1-3a or MZL who were previously treated with ≥ 1 prior systemic therapy with documented relapsed or refractory disease but not refractory to rituximab (refractory was defined as < partial response to rituximab or rituximab-chemotherapy OR disease progression < 6 months after last rituximab dose). Patients were stratified by prior rituximab treatment (yes vs no), time since last antilymphoma therapy (≤ 2 vs > 2 years), and histology (FL vs MZL), and then randomized 1:1 to R2 or control for up to 1 year. R2 patients received oral lenalidomide 20 mg/day (d), d1-21/28 for 12 cycles plus rituximab IV 375 mg/m2 weekly in cycle 1 and d1 of cycles 2-5. Control patients received rituximab and placebo on the same schedule. Dose modifications were pre-specified in the protocol to manage toxicities. The primary endpoint was progression-free survival (PFS) per 2007 IWG criteria without PET as assessed by Independent Review Committee (IRC; central review). Secondary endpoints included overall response rate (ORR), complete response (CR), duration of response (DOR), time-to-next antilymphoma treatment (TTNLT), overall survival (OS), and safety.
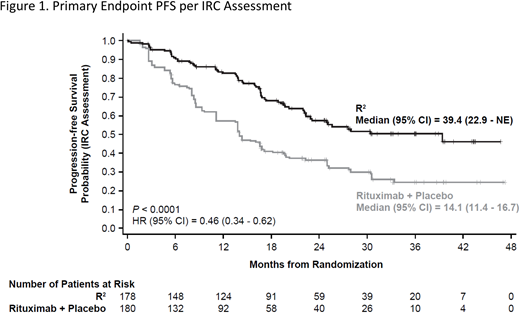
Results: A total of 358 patients were randomized (n = 178 R2; n = 180 control), median age was 63 years (range, 26 - 88), 34% FLIPI score ≥ 3, and histologies of 82% FL/18% MZL. Median number of prior systemic treatments was 1 (range, 1 - 12); 84% received prior rituximab and 51% had prior antilymphoma therapy within 2 years of enrollment. At median follow-up of 28.3 months, the study met its primary endpoint of PFS with HR = 0.46 (95% CI, 0.34 - 0.62; P < 0.0001) (Figure 1). Median PFS was 39.4 months for R2 vs 14.1 months for control. IRC-assessed ORR for R2 was 78% vs 53% for control (P < 0.0001). CR was 34% for R2 vs 18% for control (P = 0.001). Median DOR was 36.6 and 21.7 months for R2 and control arms, respectively. TTNLT was improved for R2 vs control with HR = 0.54 (95% CI, 0.38 - 0.78; P = 0.0007). OS data were not mature with 16 deaths reported in the R2 arm vs 26 deaths in control (HR = 0.61 [95% CI, 0.33 - 1.13]). Selected all-grade treatment-emergent adverse events (TEAEs) of interest more common in the R2 vs control arm (≥ 10% difference) were infections (63% vs 49%), cutaneous reactions (32% vs 12%), constipation (26% vs 14%), thrombocytopenia (15% vs 4%), and tumor flare reaction (11% vs 1%). Grade 3/4 TEAEs were reported in 69% R2 and 32% control patients. More frequent grade 3/4 TEAEs in the R2 vs control arm were primarily attributable to increased neutropenia (50% vs 13%) and leukopenia (7% vs 2%). Grade 5 TEAEs were reported in 2 patients in each arm. TEAEs leading to discontinuation of lenalidomide occurred in 9% of patients vs 5% for rituximab + placebo. Neutropenia was the only TEAE leading to discontinuation of lenalidomide in > 1 patient (n = 5). Seventy-one percent of R2 patients completed all 12 cycles of planned treatment vs 61% of control. Disease progression was the leading reason for discontinuation of lenalidomide/placebo (n = 21 R2, n = 54 control).
Conclusions: R2 demonstrated superior efficacy over rituximab monotherapy (plus placebo) as measured by the primary endpoint of progression-free survival as well as secondary endpoints of ORR, CR, DOR, and TTNLT in patients with R/R FL grade 1-3a and MZL. At this early analysis, fewer deaths have been observed in the R2 arm. Despite additional hematologic toxicity, greater efficacy of the R2 regimen (and fewer early progressions) allowed more patients to complete the planned therapy and delayed the need for subsequent treatment. R2 represents an important new treatment option in patients with previously treated FL/MZL, with meaningful advantages over single-agent rituximab.
Leonard:Celgene: Consultancy; Karyopharm: Consultancy; ADC Therapeutics: Consultancy; MEI Pharma: Consultancy; Juno: Consultancy; Bayer: Consultancy; AstraZeneca: Consultancy; Sutro: Consultancy; Biotest: Consultancy; United Therapeutics: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Genentech/Roche: Consultancy; Gilead: Consultancy; BMS: Consultancy. Trněný:F. Hoffman-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Abbvie: Honoraria, Research Funding; Gilead: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Morphosys: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Sandoz: Honoraria. Izutsu:Otsuka: Honoraria; Bristol- Myers Squibb: Honoraria; Nihon Medi-Physics: Honoraria; Novartis: Honoraria; Mundhi: Honoraria; HUYA Bioscience International: Research Funding; Kyowa Hakko Kirin: Honoraria; Takeda: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Amgen: Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Gilead Sciences: Honoraria; Eisai: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Zenyaku: Research Funding; Celltrion: Research Funding; MSD: Honoraria; Ono: Honoraria, Research Funding; Symbio: Research Funding; Celgene: Consultancy, Research Funding; Solasia: Research Funding; Sanofi: Research Funding; Meiji Seika: Honoraria; Shionogi: Honoraria; Asahi Kasei: Honoraria. Fowler:Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Zhu:Beijing Cancer Hospital (Peking University Cancer Hospital): Employment. Scheliga:INCA - Instituto Nacional Do Cancer, Brazil: Employment. Pinto:Servier: Consultancy; BMS: Honoraria, Research Funding; MSD: Honoraria; Roche: Honoraria; Takeda: Honoraria; Celgene: Honoraria; Gilead: Honoraria. Scheinberg:Novartis: Consultancy, Speakers Bureau; Janssen: Honoraria, Research Funding; Pfizer: Speakers Bureau. Flinn:Trillium: Research Funding; Takeda: Research Funding; Calithera: Research Funding; Incyte: Research Funding; Verastem: Research Funding; ArQule: Research Funding; Janssen: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding; Forty Seven: Research Funding; Agios: Research Funding; BeiGene: Research Funding; Kite: Research Funding; Portola: Research Funding; Verastem: Consultancy, Research Funding; Celgene: Research Funding; Pfizer: Research Funding; Forma: Research Funding; Merck: Research Funding; Novartis: Research Funding; Constellation: Research Funding; Curis: Research Funding; Infinity: Research Funding; TG Therapeutics: Research Funding; Genentech: Research Funding; Gilead: Research Funding. Moreira:Instituto Português de Oncologia do Porto FG, EPE, Porto, Portugal: Employment. Liu:Celgene: Employment, Equity Ownership. Kalambakas:Celgene: Employment, Equity Ownership. Fustier:Celgene: Employment, Equity Ownership. Wu:Celgene: Employment, Equity Ownership. Gribben:Cancer Research UK: Research Funding; Unum: Equity Ownership; Novartis: Honoraria; Abbvie: Honoraria; Wellcome Trust: Research Funding; Acerta Pharma: Honoraria, Research Funding; Kite: Honoraria; NIH: Research Funding; Roche: Honoraria; Janssen: Honoraria, Research Funding; Medical Research Council: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; TG Therapeutics: Honoraria; Pharmacyclics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal